TSI Biofluid Dynamics Technology
Our technology extracts 180,000 hemodynamic and pneumodynamic markers from time series data by using a biofluid dynamics approach. Descriptive, diagnostic, and predictive models are created from these markers with simple, rigorous statistical processes that typically require only one degree of freedom. Our approach is unrelated to ML in that it places the primary emphasis on discovering the most relevant fluid dynamics rather than on fitting the data. Having accomplished the former, the latter becomes fairly trivial. It also requires much less data and data processing. We can collect data through auscultation, PPG and NIR devices. All of our clinical studies (right) have utilized auscultation data from ordinary mobile phones.
Planned new phone research includes: 1) reproducing entire echo and spirometry reports, 2) detecting volume overload, 3) predicting unfavorable pregnancy outcomes.
Intended uses of the technology are listed below. In remote applications, patients would self-examine with their own phones and results would be sent to providers through an existing integrator. However, many other loT devices, such as stethoscopes, could be enabled for use by providers in clinical settings.
Remote Patient Monitoring: chronic disease patients would be baselined in clinical offices and then monitored remotely for changes during treatment, post-discharge, or over long periods of time. Pregnancy monitoring utilizes similar hemodynamics and our research suggests that health unfolds as characteristic “trajectories” that are both diagnostic and predictive. Trajectory models could be diagnostic/predictive in chronic disease.
Gold Standard Proxy: the technology can proxy a wide range of gold standard tests for use when none is available including telehealth, EMS and low resource environments. It can also evaluate VO2 Max in resting patients which expands its clincal utility to handicapped, older and medically fragile patients.
Personalized Medicine: in clinical trials, models would be created that tie outcomes to specific biofluid dynamics. Model-enabled devices would then be marketed, along with the new intervention, to providers for much more robust determination of patient eligibility and effective treatment planning.
Medical Research: our biofluid dynamics markers can phenotype patients, classify disease and model response in ways not possible with any other technology. In phase 1 clinical trials, better understanding of pharmacodynamics/pharmacokinetics could be achieved by more frequent and comprehensive monitoring.
Medical Counter Measure: based on our research with BARDA, phone based models could be used to detect new influenzas in remote populations for vaccine development. Diagnostic models could also be trained in response to a wide range of new infectious and toxic threats rapidly and with small sample sizes.
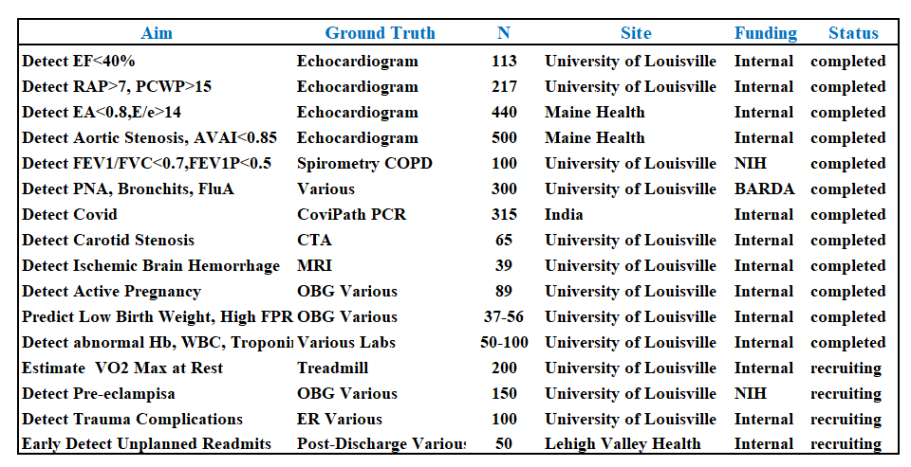
Selected clinical studies to-date: